Table of Contents
Activity 4.5 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.5 asks us to add alkaline potassium permanganate in warm ethanol and observe the change in colour.
Observation:
Initially red colour of potassium permanganate disappear. On adding more potassium permanganate red colour prevail.
Explanation:
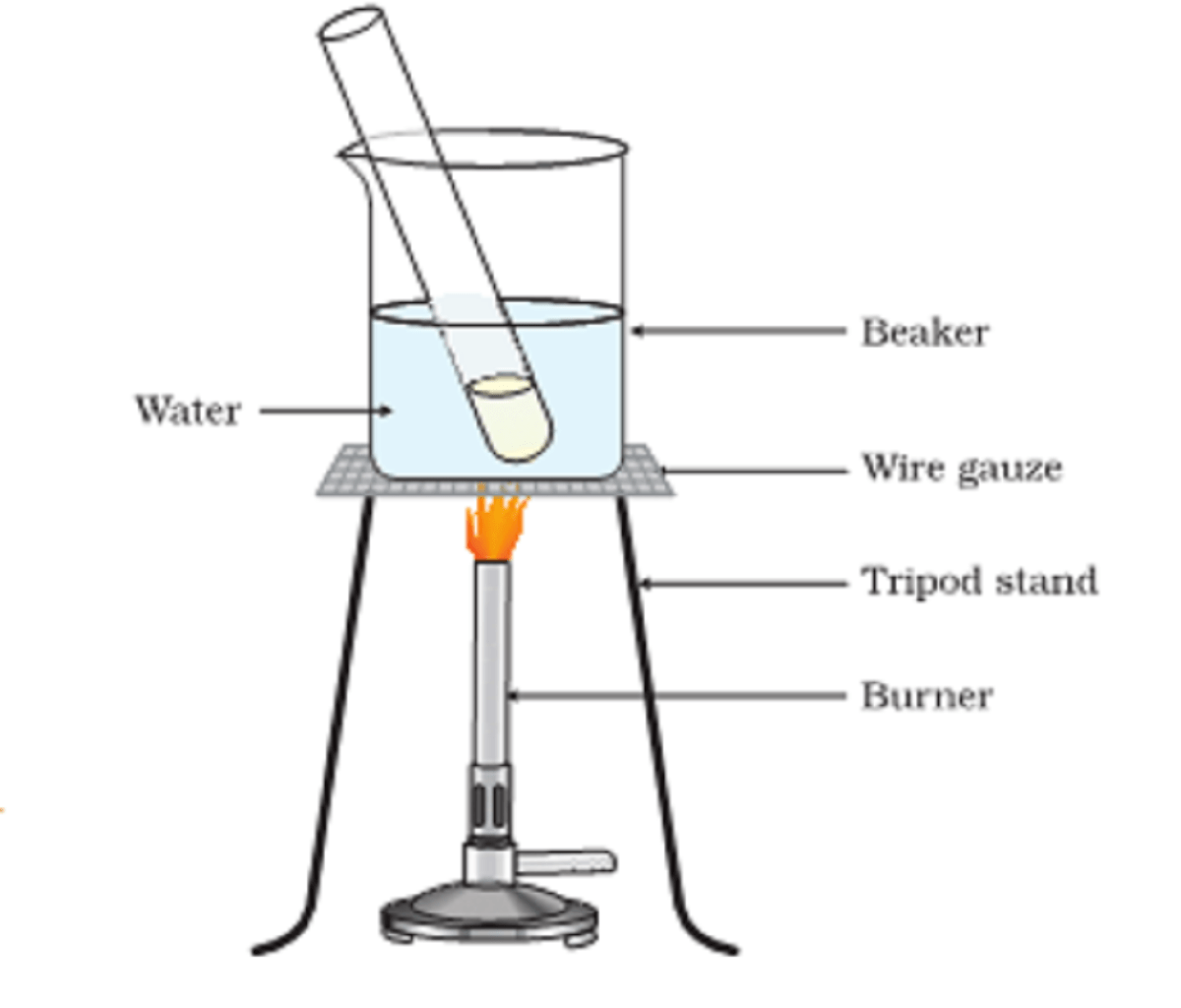
Why we use a water bath?
Alcohol is a highly inflammable substance. Heating it directly may lead to an explosion. A water bath is a beaker filled with water. We add the alcohol in a test tube and place it in a beaker. It warms the solution without catching fire.
Next: Reaction of ethanol with sodium. Activity 4.6.
See also: Bunsen Burner design and functions of its components, Activity 4.4.
Please add conclusion
Thank you sir! It helped me a lot.
Awesome explanation.
sir you are great.