Table of Contents
Activity 4.8 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.8 asks us to react glacial acetic acid with ethanol in the presence of acid and observe the smell.
Observation:
We observe a fruity smell from the solution.
Explanation:
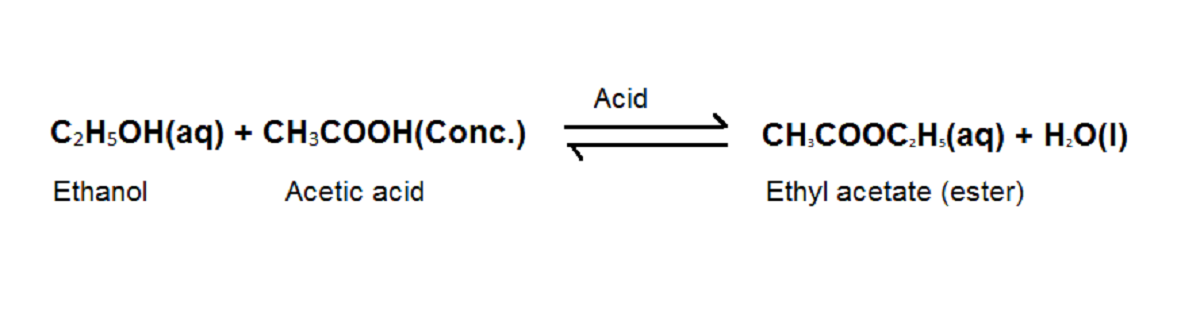
This is a reversible reaction, ethyl acetate produced is dilute after formation to avoid the backward reaction. Addition of bases like sodium hydroxide also reverses the reaction.
Next: Activity 4.9 – Reaction of ethanoic acid with carbonates.