Table of Contents
Activity 4.4 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.4 asks us to examine the flame colour with changing the position of the knob of the Bunsen burner.
Observation:
The flame changes its colour from yellow to blue with the change in position of the knob.
Explanation:
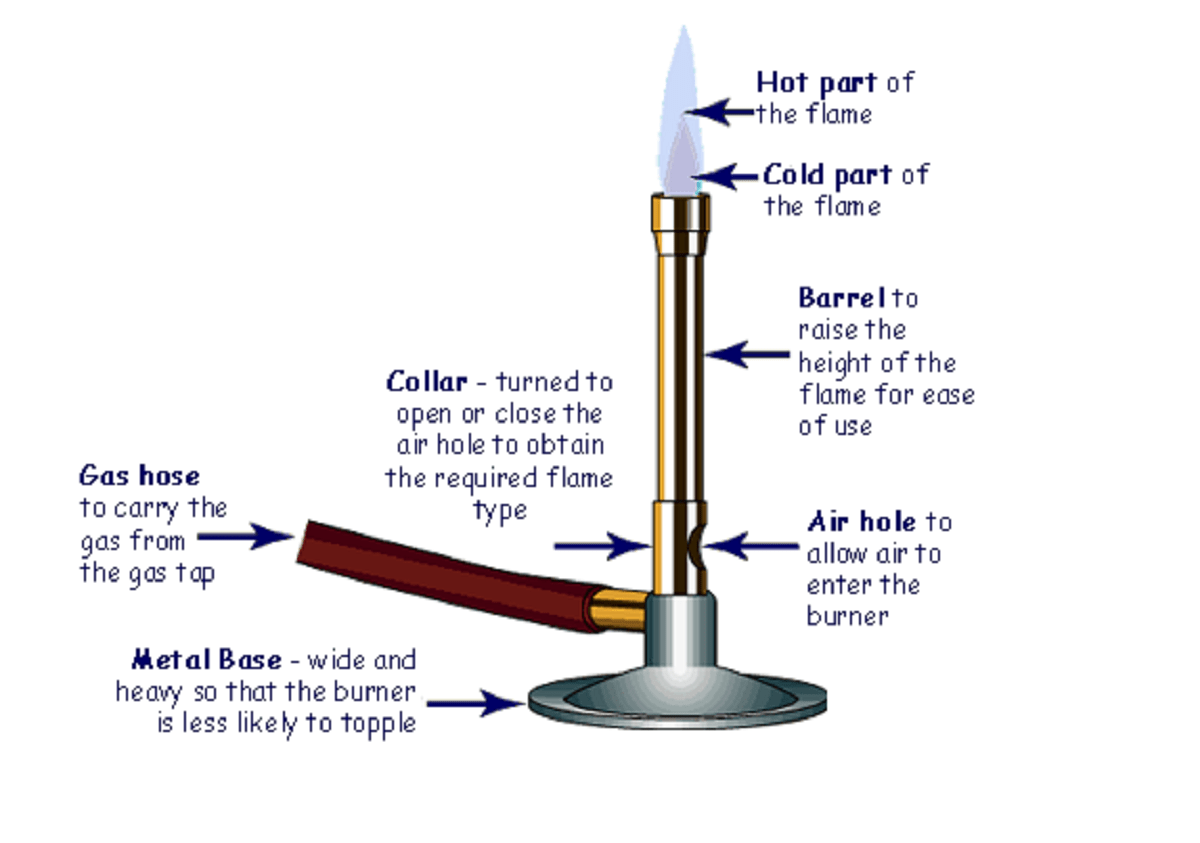
Bunsen burner is a simple burner which we use in a laboratory.
It consists of a gas pipe, a metal base, barrel and an adjustable collar.
At the bottom of the flame pipe, there are two small holes for air. A small knob, Collar is present near the hole to adjust the air inflow.
If the knob is completely open flame is blue and of smaller height. While if we close the knob the flame height increase with a yellow flame.
Gas is the common LPG gas that we use in daily life. LPG (liquified petroleum gas) is a carbon compound. It is butane the fourth homologous of the simplest carbon compound, methane. Under the insufficient quantity of air, it burns with a yellow flame.
Next: Oxidation of primary alcohol with Alkaline Potassium permanganate. Activity 4.5.
See also: Burning of organic compounds in air, Activity 4.3.