Activity 4.3 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.3 asks us to burn various organic compounds and observe the flame.
Observation:
Naphthalene and camphor burn with yellow flame while alcohol burn with a blue flame.
Explanation:
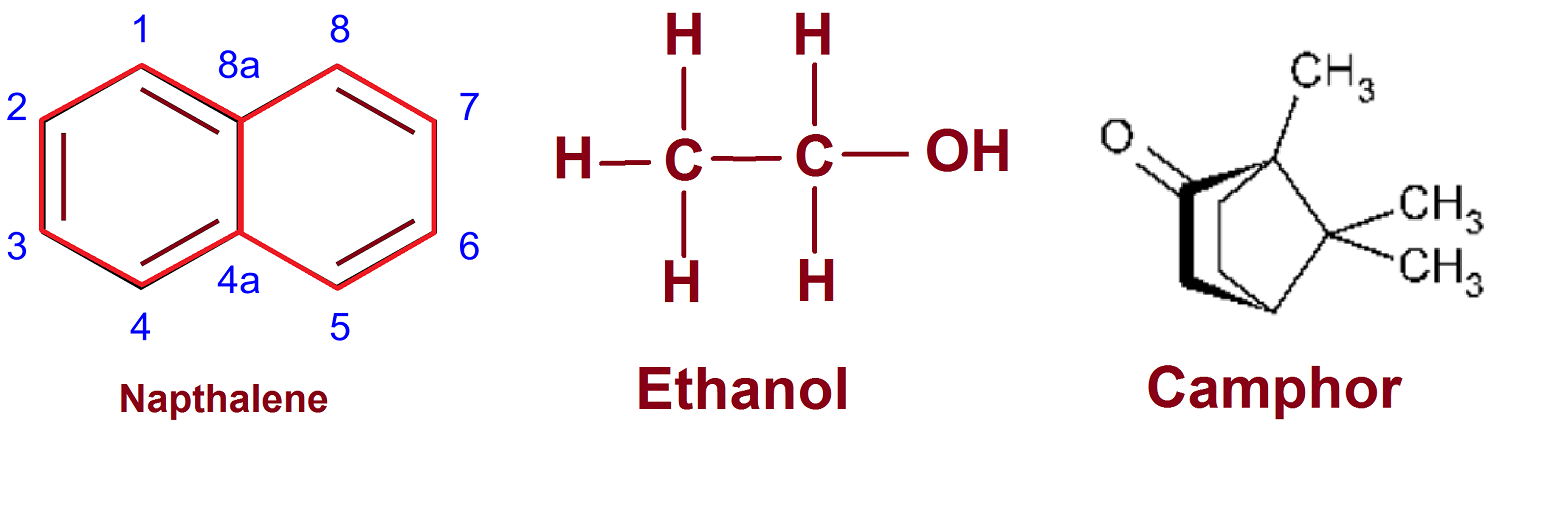
We use Naphthalene to preserve cloth from microorganisms. It is an unsaturated compound with multiple double bonds. It burns in air and gives yellow smoke.
Alcohol and camphor are saturated hydrocarbon. Alcohol burns with a blue flame which show complete combustion. Camphor burn with a yellow flame.
Colour of the flame depends on the amount of carbon relative to others. The higher proportional carbon atom results in incomplete combustion. Naphthalene has five double bonds so it has more carbon atoms; camphor has one cyclic ring so lesser than naphthalene; while ethanol is completely saturated carbon compound without a cyclic ring. As a result, naphthalene has a sooty yellow flame while ethanol has a blue flame.
Next: Details of Bunsen Burner Activity 4.4.
See also: Homologous series definition and properties, Activity 4.2.
plz explain the topics too side by side.