NCERT Class 9 Science Chapter 1 Matter in our Surroundings Exercise solution
1. Convert the following temperatures to the Celsius scale.
(a) 293 K (b) 470 K.
Answer:
°C = temperature in Kelvin – 273
so, 293 K = 20°C
470 K = 197°C
2. Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C.
Answer:
Temperature in Kelvin (K) = °C + 273
so, 25°C = 298K
373°C = 646K
3. Give the reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answer: a) Solid, liquid and gaseous state of a substance always remain in equilibrium with each other. Some substances like naphthalene, camphor have narrow liquid phase. They sublimate and form a vapour.
b. Particles in a gaseous medium have high kinetic energy. They disperse in the surrounding fast. As a result, we can detect the smell of perfume from a far away distance.
4. Arrange the following substances in increasing order of force of attraction between the particles— water, sugar, oxygen.
Answer: We can easily compare the force of attraction between molecules of different states. A solid has the highest force of attraction and gas have the lowest. So the force of attraction here will be:
Sugar>Water>oxygen.
5. What is the physical state of water at—
(a) 25°C (b) 0°C (c) 100°C ?
Answer:
25°C is a reference to room temperature. At this temperature, water remains in the liquid state.
0°C is the freezing point of the water. At this point, water remains in equilibrium with ice.
100°C is the boiling point of water. At this point, water remains in equilibrium with vapour form.
6. Give two reasons to justify—
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answer:
a)Freezing point, melting point and boiling point of a substance are characteristic of that substance. Water below 0°C remains in solid form. Beyond 100°C, it stays in gaseous form. We take the average room temperature at 25°C. At this temperature, water remains in the liquid form.
b)Iron has a melting point of 1,538 °C. This temperature is far from room temperature. As a result, an iron almirah is a solid at room temperature.
7. Why is ice at 273 K more effective in cooling than water at the same temperature?
We get the cooling effect of water because water loses its energy to change into its vapour form. This loss is in the form of latent heat of vaporisation(Lv). Ice spends its energy and converts itself into the water first. Here the energy consumed is the latent heat of fusion (Lf). Now again formed water spends its energy in the form of Lv. So if we compare the cooling effect of water and ice, Ice has always more cooling effect at any temperature.
8. What produces more severe burns, boiling water or steam?
Answer:
Steam contains energy due to its temperature as well as latent heat of vapourisation. When a body cell comes in contact with the steam, the cells of the body receive high energy. As a result, exposure to steam is more dangerous.
The energy of stem = Kinetic energy due to its temperature + Latent heat of Vapourisation.
Boiling water has only kinetic energy due to its temperature. Hence, here damage to cells is less.
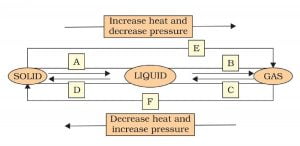
9. Name A, B, C, D, E and F in the following diagram showing the change in its state
A: Melting. e.g. Ice to water.
B: Boiling, e.g. water to vapour.
C: Condensation. e.g. Vapour to water.
D: Freezing. e.g. Water to ice.
E: Sublimation. e.g. Burning of camphor.
F: Deposition. e.g. Deposition of camphor in Activity 1.13.
Next: Is Matter Around us Pure Solution.
See also: Activity solution: Matter in Our surrounding.