Activity 1.12 NCERT Science Class 9 Chapter 1 Matter In Our Surroundings
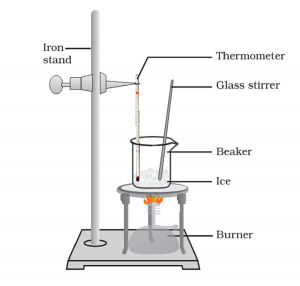
Brief procedure:
Activity 1.12 asks us to heat ice on a water bath and record its temperature at which it starts melting. Similarly, it also want to heat a water solution so that it starts boiling.
Observation:
We see that at 0°C ice starts melting and this temperature remains constant till all the ice melts.
During the boing of water, we see that the temperature of the water remains stable at 100 °C.
Explanation:
Why does ice melt?
There is a direct relationship between the kinetic energy of the molecule and the temperature. If we raise the temperature, the kinetic energy of the molecules also increases. A similar thing happens with the water molecule also. When we heat the ice cubes, ice cubes take the heat energy and increases its kinetic energy. If the heating is sufficient, then the kinetic energy of the water molecules increases to such an extent that ice converts into water.
Why the temperature remains stable at 0 °C?
O °C is an equilibrium point for the conversion of ice into water. Any increase in temperature at this point is used by the water molecules to break the force of attraction. Since we can not see any change in the temperature, we call this energy as latent heat, “latent heat of fusion.”
Conversion of water into vapour
When we continue heating the water, at 100°C water molecule again break the force of attraction and become vapour. Here, 100°C acts as an equilibrium point, and any energy beyond this is utilised to break the force of attraction. So the temperature also remains the same, i.e. 100°C. We call this latent heat as latent heat of vaporisation.
Inference/conclusion:
This experiment demonstrates that we can change the physical state of matter by heating.
Solid —> Liquid —-> Gas
Next: Experiment to demonstrate sublimation and deposition, Activity 1.13.
See also: Experiment to demonstrate a gas is compressible: Activity 1.11.
Application:
Coal thermal plant: In a thermal plant we heat water to produce steam. The steam rotates the turbine which produces electricity. See how a thermal plant work.
Copper idol: A coppersmith melts the copper at high temperature to put the molten copper into moulds of an idol.
Sterilisation of surgical equipment: In the laboratory, we heat surgical equipment in an appliance similar to a pressure ‘autoclave‘. Here Latent heat of vaporisation kills the germ very fast.