Activity 1.13 NCERT Science Class 9 Chapter 1 Matter In Our Surroundings
Brief procedure:
Activity 1.13 asks us to heat camphor or ammonium chloride into a beaker and observe what happens.
Observation:
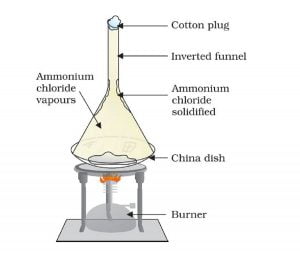
Heating camphor or ammonium chloride result in its vapour formation. These vapour cool at the upper part of the beaker and deposit there.
Explanation:
Some substances have a low melting point range. Such solids on heating at low pressure convert into its gaseous form without undergoing into liquid form. Here camphor or ammonium chloride has a low melting point range. Upon heating, these substances directly transform into its vapour form. We call this process Sublimation.
Cotton plug on the beaker prevents the escape of the formed vapour. In the upper part of the beaker, the temperature is lesser. As a result, the vapours cool down and deposit as solids on the neck of the beaker. This process of conversion of gas to solid is inverse of sublimation and we call this process Deposition.
Inference/conclusion:
This experiment demonstrates the process of sublimation and deposition.
This experiment shows that camphor and ammonium chloride can be easily sublimated and deposited.
We use the term “triple point” to demonstrate sublimation. It is the temperature and pressure where all the forms of a substance 1.e. solid, liquid and gas exist in equilibrium. A triple point is fixed for a substance. For water, it is 0.01 °C and 4.5mm Hg of pressure. A substance with a narrow liquid phase easily sublimates.
Some common easily sublimated substance other than camphor or ammonium chloride is Dry carbon dioxide ( dry ice), and Naphthalene. Read more: Wikipedia.
Next: Experiment to demonstrate the change in the rate of evaporation, Activity 1.14.
true
It’s аn awesome post in faѵor of all the online users; they will take advantage from it I am sure.