Science Class 9 Chapter 3 Atoms & Molecules Exercise Solution
1. A 0.24 g sample of a compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
answer:
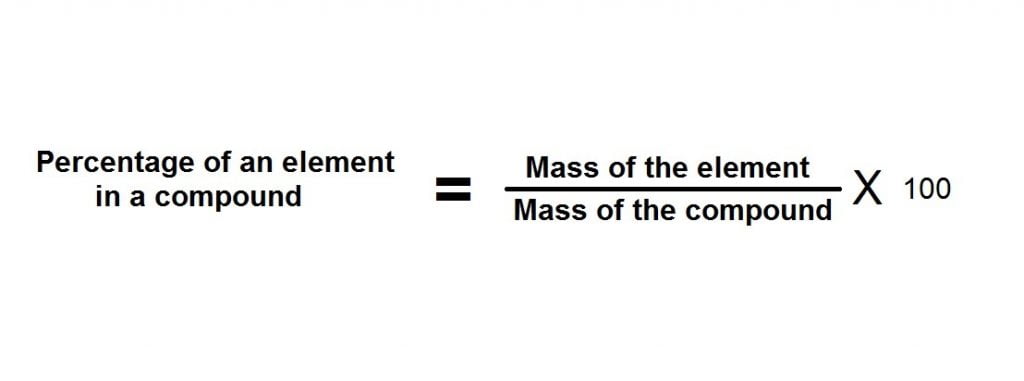
Percentage of boron in the sample = (0.096/0.24)*100 = 40%
and the percentage of oxygen in the sample = (0.144/0.24)*100 = 60%
Hence, the percentage combination of boron and carbon in the given sample is 40 an 60% respectively.
Alternatively, we can use unitary law to find the answer as follows:
Since 0.24 gm of the sample contain 0.096gm of boron.
So, 1 gm of sample contain = 0.096/0.24 gm of boron.
Therefore, 100gm of the sample contains = (0.096/0.24)*100 = 40%
Similarly,
Since 0.24 gm of the sample contain 0.144 gm of oxygen.
So, 1 gm of sample contain = 0.144/0.24 gm of oxygen.
Therefore, 100gm of the sample contains = (0.144/0.24)*100 = 60%
2. When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Answer:
As per the law of constant proportion, the ratio of masses of elements in a compound is in definite proportion. Here 3 gm of carbon will take 8 gm of oxygen and will form 11 gm of carbon dioxide. The remaining oxygen will be in unused form.
Next: Question 3: What are polyatomic ions? Give examples.