Activity 1.14 NCERT Science Class 9 Chapter 1 Matter In Our Surroundings
Brief procedure:
Activity 1.14 asks us to put water in different types of vessels, and compare the rate of evaporation with the change in temperature and humidity.
Observation:
Water in vessels with a larger surface like china dish area evaporates faster.
When we compare this with summertime, the rate of evaporation is much higher.
In the rainy day, the rate of evaporation is the lowest.
Explanation:
Water at its surface is always in equilibrium with its vapours because some of the molecules have higher kinetic energy and they escape from the surface. We call this process Evaporation. Presence of wind or increase in temperature promotes this process while humidity lowers the escape.
- As a result, when we keep water in vessels with the different surface areas like a test tube and a bowl or china dish; the rate of evaporation is higher in vessels with a large surface area.
- In summer the temperature is higher than in the winter. As a result, water has more kinetic energy than the normal days. This high kinetic energy results in a higher rate of evaporation in summer.
- A humid environment like a rainy day shifts the equilibrium in the backwards side. As a result, we see a lesser rate of evaporation.
Uses:
We spread our water-soaked cloths in sunlight or below a fan to dry it faster.
Application:
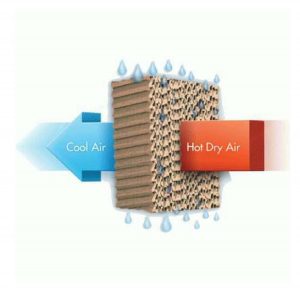
Evaporation is an endothermic process. The energy here is utilised to the latent heat of vapourisation. As a result, energy in water lowers and it becomes colder. We use this property in the desert coolers in houses. The high surface area of evaporative pad (wool, paper, khas-khas) lowers the temperature of the water. So, when the fan blows wind from them, we get more refreshing air.
Why desert cooler work best in summer days than regular days.
Here also the rate of evaporation comes into play. In summer days the rate of evaporation is higher than regular days. So the difference between normal air and air through the cooler is high.
In winter the rate of evaporation is slow. So the cooler does not make a big difference.
Other activities from chapter 1 Matter in our surroundings.
Thanks! It helped me a lot
Plz explain it in simple words