Table of Contents
Activity 4.9 NCERT Class 10 Science, Carbon, and its Compounds
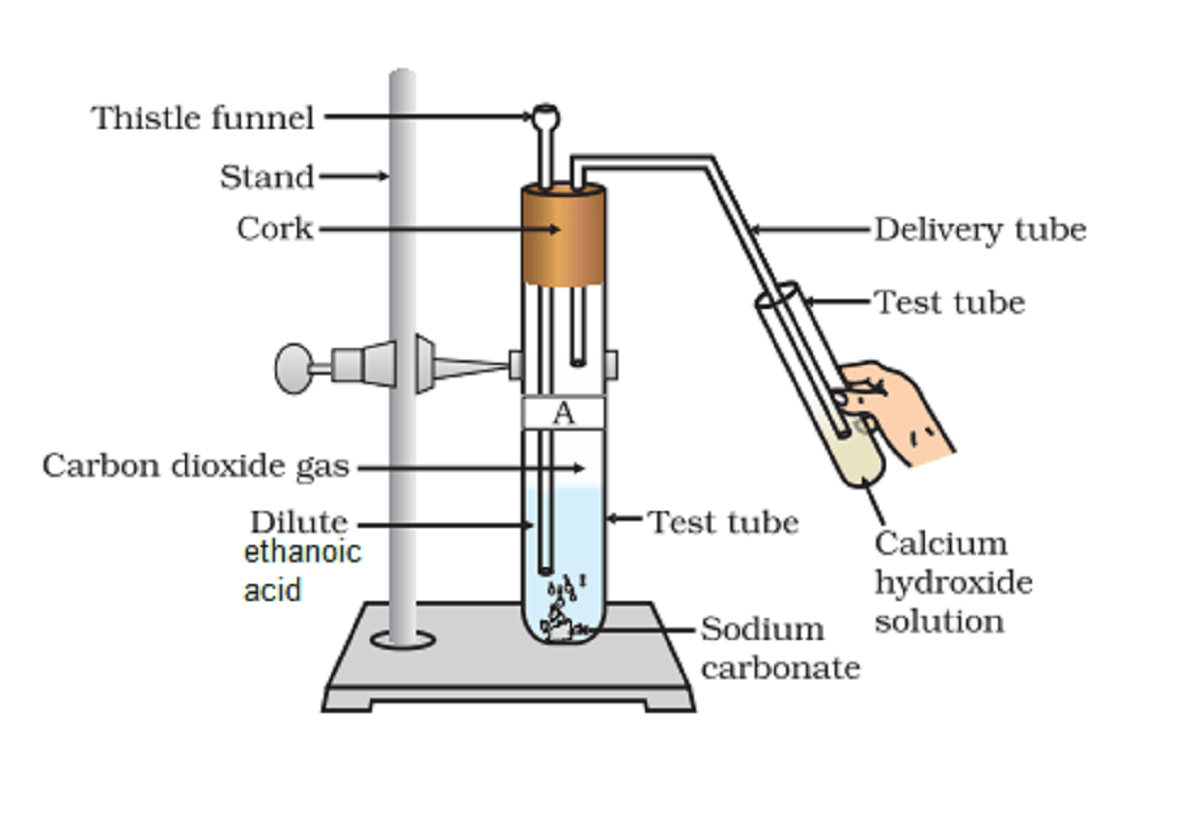

Activity 4.8 asks us to see if there is a reaction between ethanoic acid and carbonates or hydrogen carbonate like Activity 2.5.
Observation:
Similar to inorganic acids, ethanoic acid reacts with sodium carbonate and hydrogen carbonates. Evolved gas turns the lime water solution milky.
Explanation:
Carboxylic acids react with carbonates and hydrogen carbonate in the same fashion of inorganic acids and form respective salts. The only difference is the speed of the reaction. Here reaction takes place slowly.
Here sodium metal replaces the most polar hydrogen atom and forms the salt. Carbon dioxide gas evolves which turns the lime water milky.
Next: Activity 4.10 – Soap action on dirt and oil.
Explain properly