Activity 4.11 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.11 asks us to check the amount of foam produced by soap in hard water and compare it with foam produced by soap in distilled water.
Observation:
We see more amount of foam in soap with distilled water.
Explanation:
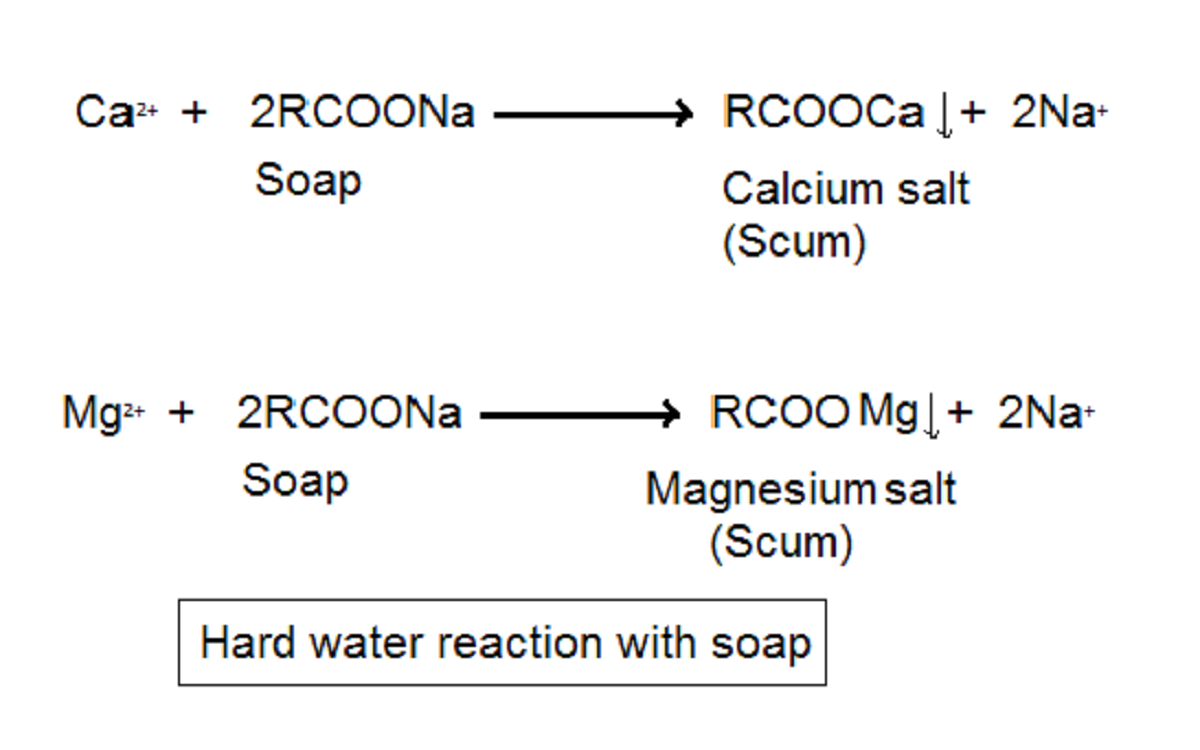
Hard water contains some amount of hydrogencarbonates, sulphates, and chlorides of calcium and magnesium. These salts displace sodium from the soap and form fatty acid of magnesium and calcium. These salts are insoluble and deposit on the bottom as scum. As a result, less soap is available to form the foam
Next: Difference between soap and detergent. Activity 4.12
Easy to understand and it’s clearly explained
this is helpful for me