Activity 3.1 NCERT Class 9 Science Chapter 3 Atoms and Molecules
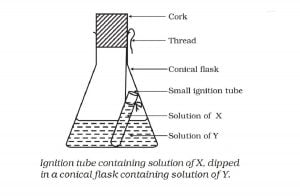

Activity 3.1 asks us to boil the combination of solutions and see:
- If heating them separately cause any change in mass.
- If mixing of the solution changes the mass.
Observation:
Heating the solution separately or mixed does not change the mass.
Mixing the solution result in chemical reactions but the mass of the flask remains the same.
Explanation:
Mass of the sum of reactants and the products during a chemical reaction remain constant. For example, if we react 23 grams of Sodium and 35.5 gm of chlorine (23 and 35.5 gm correspond to molecular weight), the resultant sodium chloride will be 58.5 gm always.
Here, when we heat the mixture separately, no reaction takes place. Since flask is closed by the cork, the vapours of water molecule do not escape and condense back to the bottom. As a result, we see no change in mass.
When we swirl the conical flask, the mixture from the ignition tube comes out and mix with the solution in the flask. It results in the chemical reaction given below:
- Copper in copper sulphate displaces sodium from its carbonate and releases carbon dioxide. There is a change in the colour, the blue solution of copper sulphate change to green.
- Barium chloride reacts with sodium sulphate and forms a white precipitate of barium sulphate.
- Lead nitrate reacts with sodium chloride and forms a white precipitate of lead chloride and an aqueous solution of sodium nitrate.
Mass of the substance is constant in all these reactions.
Next: Activity to demonstrate the law of definite proportion, Activity 3.2.
See also: Activities from the 3rd chapter.
Please tell how to make 5 % of solution