Table of Contents
Activity 1.10 NCERT Science Class 10 Chemical Reactions and Equations
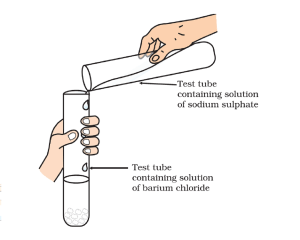
Brief Procedure:
Activity 1.10 asks us to mix the sodium sulphate solution with barium chloride solution.
Observation:
A white precipitate of barium sulphate sinks at the bottom.
Explanation:
Sodium sulphate and barium chloride undergo double displacement and form barium sulphate and sodium chloride. The reaction goes in the forward direction as barium sulphate formed is insoluble in water and sinks to the bottom.
Na2SO4 (aq)+ BaCl2(aq) —> BaSO4(white ppt.) + 2NaCl(aq)
Inference:
Sodium sulphate and barium chloride undergo double-displacement reaction and form insoluble white barium sulphate precipitate.
Next: Heating of copper powder in a china dish: Activity 1.11.
See also:
Displacement reaction of an iron nail in the copper sulphate solution. Activity 1.9
Hello
thanks for explaining activities
Thank you ☺️ for explaining this experiment in detail