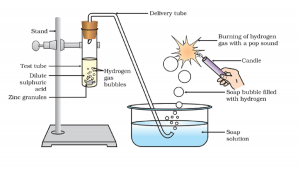
Why a reaction of a metal with acid liberates gases. And how do we detect them?
Page 22 Question 2
Acids in a solution form H+ ion. Metals are electron donor; they donate electrons to H+. Addition of electron to H+ forms a hydrogen molecule. The hydrogen molecule is a gas and does not dissolve in water. This gas escapes out.
HA(Acid) (in aqueous medium)—-> H+ + A–
M —-> M+ + e–
2H+ + 2e– —> H2(gas)
Hydrogen gas (H2) is an inflammable gas, when we bring this gas to a burner it burns spontaneously.
Next: A metal compound which reacts with acid and extinguishes the fire.
See also: Why we do not keep curd and sour substances in Brass container.