Similarities and differences between Combustion and respiration
Combustion and Respiration are similar in many ways yet different also. Let us see first what is combustion and respiration.
Combustion:
In daily life, we see numerous examples of combustion. For example, we ignite the gas stove to cook our food; a motor vehicle burns petrol and diesel to put the vehicle in motion; during winter we put wood on the fire to heat our room. In all these examples we observe that burning a substance like LPG gas, petrol and would give us the energy in the form of heat.
eg. Coal + Oxygen ———> Carbon dioxide + Energy
Metal + Oxygen ——-> Metal oxide + Energy
If we go deeper then we will find that all these processes require oxygen somehow. For example, in the inside of the burner of a stove, there are holes which allow air to come in; In a motor vehicle valves of the engine open at fix interval to let air come inside the engine. All these events show that combustion occurs in the presence of the air. So now the definition of combustion becomes; Burning of a substance in the presence of air which gives energy in the form of heat.
Respiration
All living organism needs the energy to carry out its life processes; like growth, repair of cells, movement etc. To meet this energy requirement, living organisms developed a mechanism to get the energy. In this mechanism, they selected a substance which can undergo burning easily and also liberate a good amount of energy.
Glucose + Oxygen —> Carbon dioxide + Water + Energy
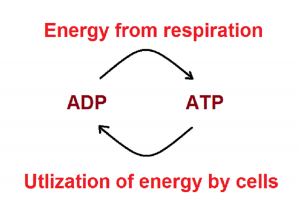
Now we can easily make similarities between the combustion and respiration.
Similarities:
- Both the process involve the burning of a substance.
- In both processes, the substance undergoes oxidation in the presence of oxygen.
- Both processes liberate energy.
- Both have by-products as oxides.
Differences
Now let us see what differences we can conclude
- Combustion is uncontrolled oxidation process, while respiration is catalyst mediated controlled oxidation.
- Any substance which can burn leads to combustion; while respiration uses glucose to give pyurvate and the to carbon dioxide.
- Combustion produces oxides in the form of smoke; in respiration there is no smoke formation.
- Product of combustion is heat energy; in respiration it is formation of ATP. AS, in the combustion heat energy is utilised instantaneously in the form of heat; while in respiration energy is trapped in the form of ATP.