Table of Contents
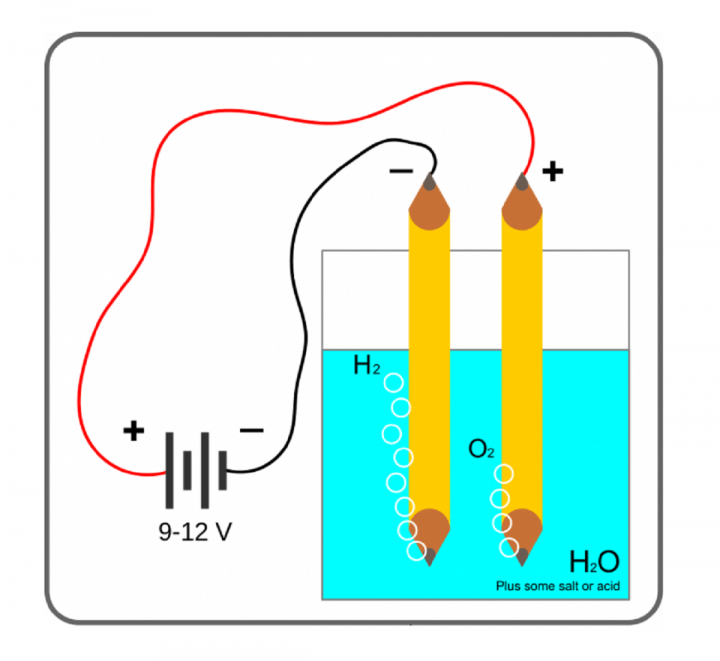
Why is the amount of gas collected in one of the test tube in Activity 1.7 double of the amount collected in other? Name the gas.
Answer:
Activity 1.7 deals with the electrolysis of water using a battery. It lyses the water into its component molecule viz. Hydrogen and oxygen.
2H2O (l) ————>2H2 (g) + 02 (g)