Table of Contents
Activity 4.12 NCERT Class 10 Science, Carbon and its Compounds
Brief procedure:
Activity 4.12 asks us to compare the foam produced by a soap with hard water and by a detergent with hard water.
Observation:
There is more foam production with detergent.
Explanation:
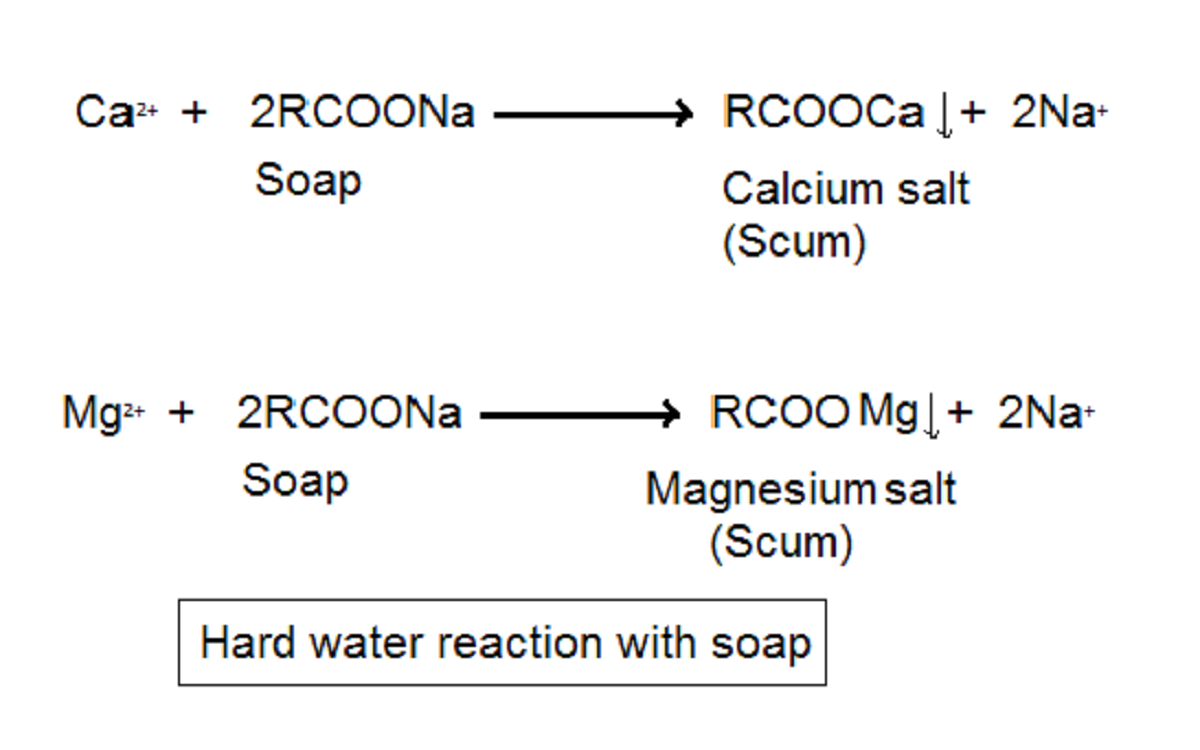
Soaps are sodium salts of naturally occurring fatty acids like palmitic acid, stearic acid etc. We prepare them by the saponification of natural oils like coconut oil, butter, olive oil with salt. They react with calcium and magnesium ions present in hard water and form an insoluble precipitate. This process reduces the quantity of soap available. Thus soap forms less foam.
Detergents are chemically synthesised compounds also known as cleansing agents. They generally consist of the sodium salt of sulphonic acid or ammonium salt with bromine and chloride ions. These do not react with ions present in hard water. As a result, there is a lesser influence of hard water on foam formation. As a result, we see more foam production with detergents than soaps.
Check other activity answers from Chapter Carbon and its Compounds.