Table of Contents
Activity 4.10 NCERT Class 10 Science, Carbon, and its Compounds
Brief procedure:
Activity 4.10 asks us to test the shake mixture of oil and water with soap and compare it with plain water and oil.
Observation:
The mixture of oil and water becomes cloudy with soap while oil separates and form a layer with plain water.
After some time the oil separates from both the solution. But it takes a longer duration for a cloudy solution.
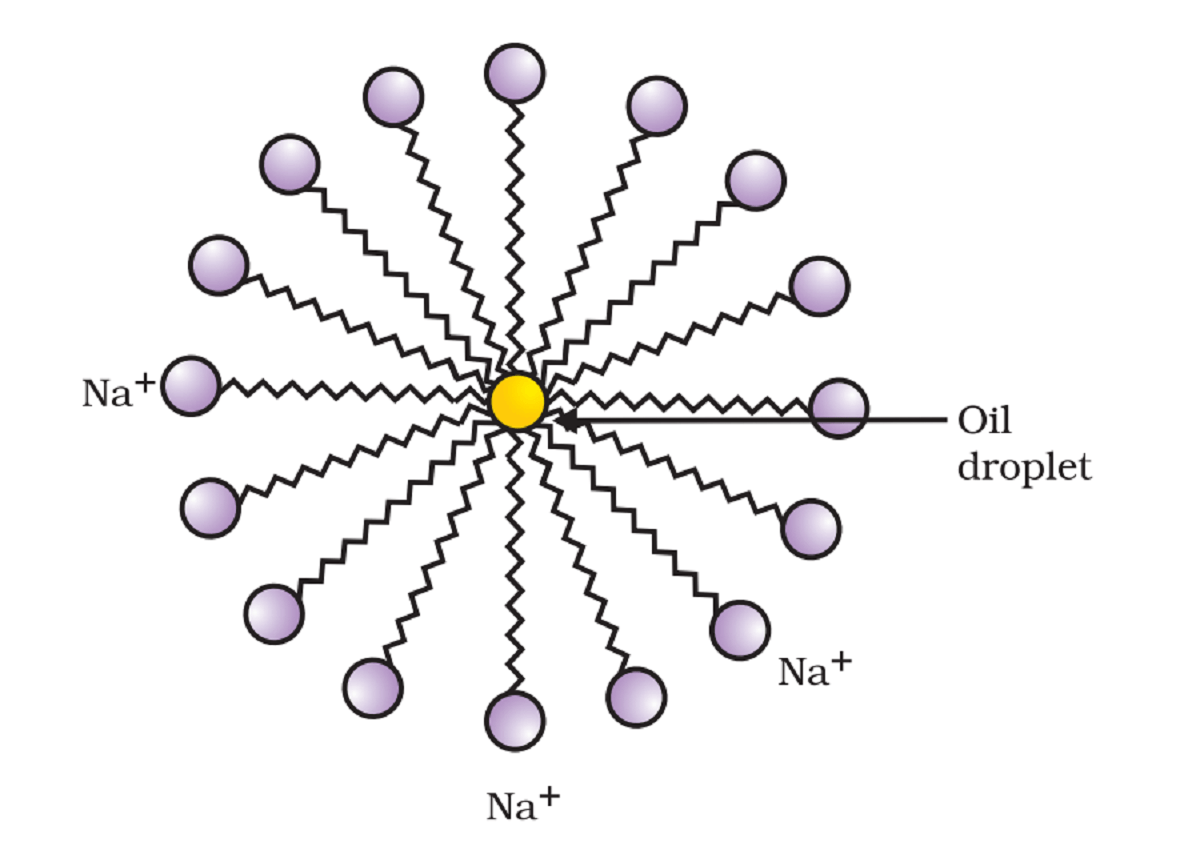
Explanation:
Water is a polar compound. A polar substance like salt forms bond with polar hydrogen and hydroxide molecules of water. This polar nature makes salt soluble in water.
Oil is a non-polar compound. It does not form bonds with water molecules. As a result, oil does not dissolve in water. Instead, they start floating on the surface of the water as they are lighter than water.
Soaps are metal salts of long chain fatty acid. It’s one end is polar (Na+) while another end is non-polar. Polar end forms bonding with the water molecule and non-polar end forms bonding with oil molecules. This form the suspension of oil in water. As a result, the solution becomes cloudy. We call this process micelle formation
Application in daily life:
Dirt in our clothes is mainly oily substances. Soap or surf suspend the dirt between water and cloth. When we agitate this solution dirt passes out from the cloth, and we get a clean cloth.
Application in biological processes:
Pancreatic lipase is a water-soluble enzyme. It can not work on the separate layer of the long chain of the fatty acid of oils. Bile salt from liver suspends the fatty acid into the water to facilitate the action of lipase enzyme. We call this emulsification of fats. You will read this in detail in chapter 6 life processes.
For more details on the role of bile, you can read the following article: What is the function of the digestive enzyme.
Next: Reaction of soap with hard water. Activity 4.11.