Table of Contents
Activity 3.14 NCERT Class 10 Science, Chapter 3 Metals and Non-metals
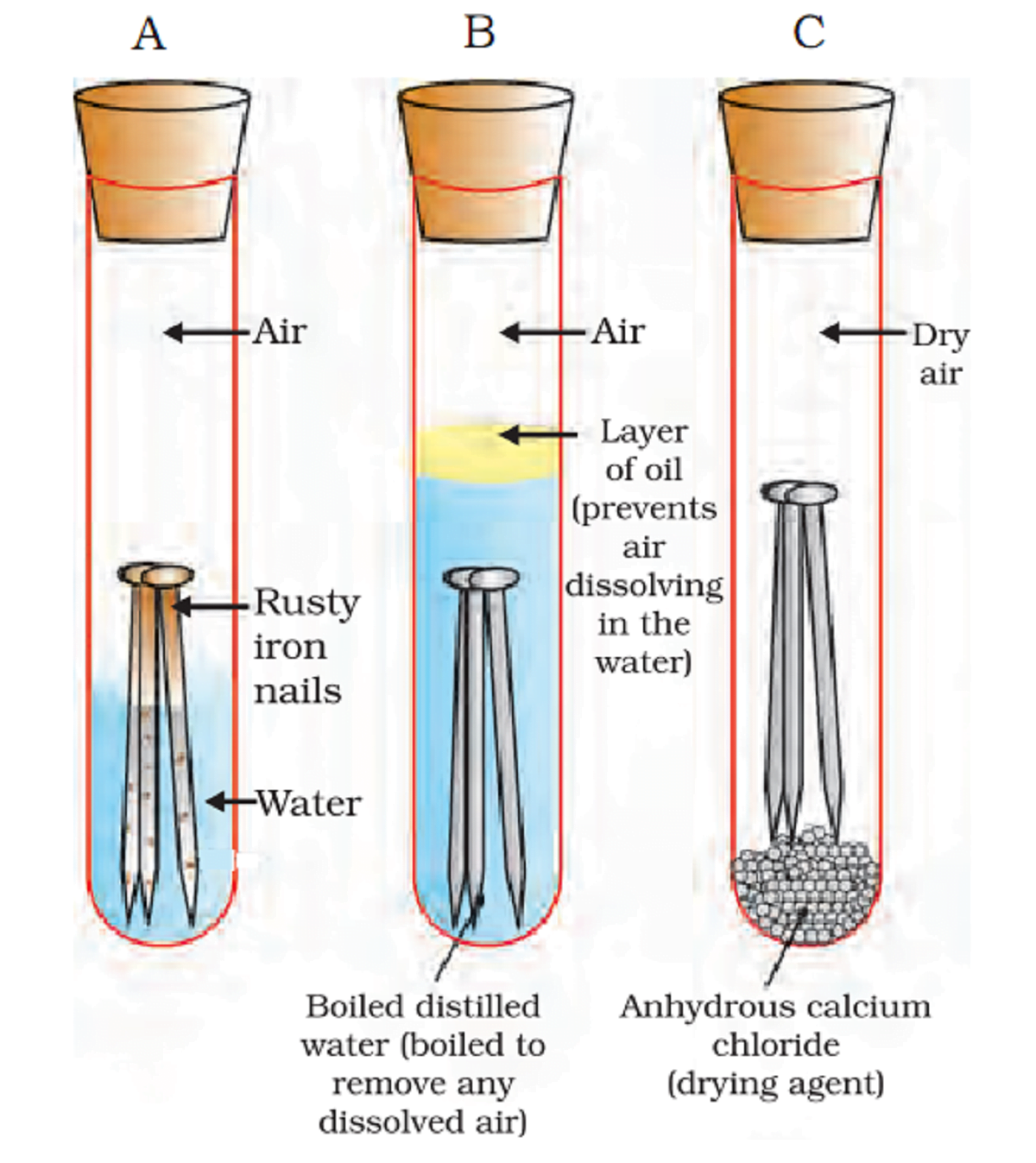

Activity 3.14 asks us to experiment with iron nails in various conditions.
A. Iron dipped in water B. Nail dipped in water with some oil floating on water. C. Iron nail with calcium chloride.
Observation:
Nails in test tube A got rusted in a few days. Nails in test tube B and C did not get rust.
Inference:
It shows water and air both are necessary to form rust.
Explanation:
Oxidation of iron metal with oxygen require high temperature. The other alternative is to use the hydration energy of water. The outer layer of iron nail reacts with oxygen in the presence of water to form its oxide.
4Fe(s) + 6H2O(l) + 3O2(g) ———-> 2Fe2O3*3H2O
Outer layer now scrapes off and give way to oxygen and moisture to the inner layer of iron. The process goes on until all iron convert into its oxide.
In the test tube, B oil prevents oxygen from dissolving in water. In the test tube C, calcium chloride acts as an absorbent and absorbs moisture present in it. So, Iron does not form rust in these test tubes.
Very good explanation
Very good website. Helps me all the time.
Very nice and useful explaination of all the chemistry practical on these site
I’m not worried about concept clearance of chemistry due to these website
Very good and breif explaination…….
Very good explanation.excellent.
I love your website !!!!!! every chemistry activity I understand by this site. It helps me a lot really! not only do i know what happens in the activities, but also I am able to save my time by not struggling at chemistry to understand the concepts ,… thanks a lot
Thanks for such a good observation and brief explanation too useful for the students.
Thanks for the awesome job of putting these activities together for class 10 student! These are immensely informative and helpful for students! hats off to you!