Table of Contents
Activity 3.12 NCERT Class 10 Science, Chapter 3 Metals and Non-metals
Brief procedure:
Activity 3.12 asks us to dip copper and iron nail into a salt solution of another metal and observe the reaction.
Observation:
More reactive metals displace with metals from other salt solution and form corresponding salts.
e.g., Iron in a copper sulphate solution.
Explanation:
Iron is more reactive than copper. It displaces copper from copper sulphate and forms ferrous sulphate. Copper sulphate solution is blue while ferrous sulphate is green. So the solution turns green from blue.
Fe(s) + CuSO4(aq) ———–> FeSO4(aq) + Cu(s)
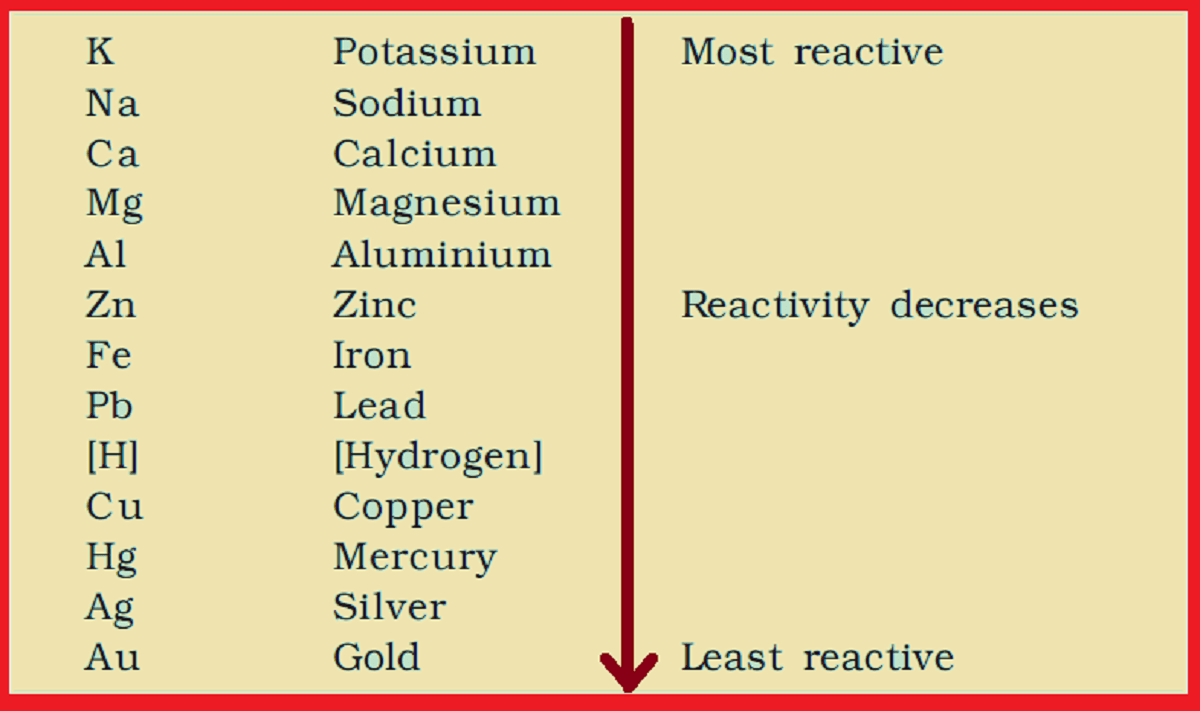
Note: The diplacement depend on reactivity series. A metal can displace a salt if the metal is higher in reactivity series.
Reactivity Series of Metals.
Next: Properties of salts: Activity 3.13.
Thankyou Soo much for this amazing explaination of each and every activity
6⏩⏩⏩❓❓❓❓❓❓⏩⏩⏩⭐⭐⭐⏩⏩⏩v997e9wf$7o1s70s70⭐⭐️️⏩️️⚡⚡⚡✨v918+381p%6-y9149y26❤️❤️❤️️❤️❤️❤️⚖️⚖️❤️❤️⚖️⚖️❤️️❤️❤️✈️❤️✈️❤️❤️❤️️️️️️️️️️️️️️️️️️️️♈♈feUe
So good explanation
Thank you
No buddy
Great explanation ! Thanks
All activities from 3.1 to 3.12 are properties of metals viz. Electrical conductivity, heat transfer, reactions with acids, malleability, ductility etc.
Can you corelate the observations with the activities 3.9, 3.10 And 3.11 ?
Can you corelate the observations with the activities 3.9, 3.10 And 3.11 ?
Explained correctly
Nicely explained