Activity 3.11 NCERT Class 10 Science, Chapter 3 Metals and Non-metals
Brief procedure:
Activity 3.11 asks us to react to various metals with dilute hydrochloric acid and observe the reaction.
Observation:
Metals react with dilute hydrochloric acid and form metal chlorides with the evolution of hydrogen gas.
Metal(s) + HCl(aq) ———–> Metal Chloride(aq) + H2(g)
E.g.
Ca(s) + 2HCl(aq) ————-> CaCl2(aq) + H2(g)
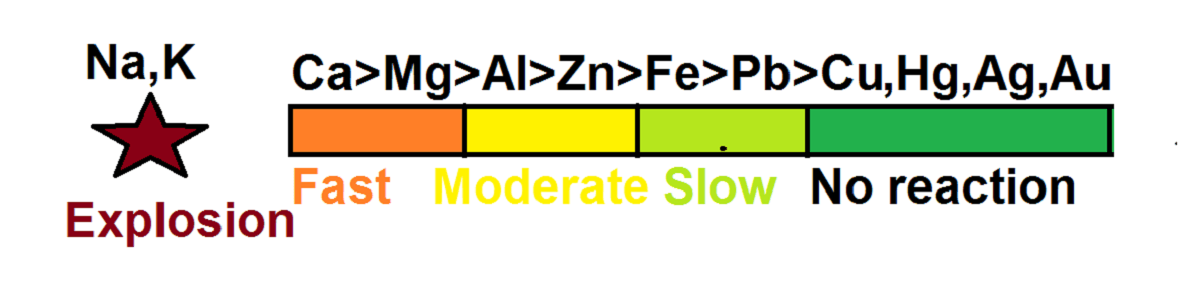
Order of reactivity:
Ca>Mg>Al>Zn>Fe>Pb>Cu
Temperature during the reaction:
Experiment done at room temperature (25˚C).
Calcium: 40˚C
Zinc: 34˚C
Iron: 30˚C
Copper: 25˚C
Inference/conclusion:
Metals react with acids to form their salt. The process is exothermic and hydrogen gas is also produced.
Next: Reaction of metal with salt, Activity 3.12.
See also: Reaction of metals with water, Activity 3.10.
Keep up the good work
It is good to write like this
Aim:
Materials required:
Procedure:
Observation:
Conclusion:
It is good to write like this
Aim:
Materials required:
Procedure:
Observation:
Conclusion:
Very helpfull
Thank you!!! It was really helpful❤