Activity 2.8 NCERT Class 9 Science Chapter 2 Is Matter Around Us Pure
Brief Procedure:
Activity 2.8 asks us to separate a mixture of acetone and water using the distillation method.
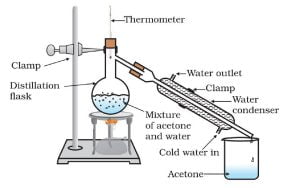
Observation:
Acetone is a volatile liquid. It easily vaporises on heating. On cooling, acetone again converts into its liquid form. Here we receive pure acetone in the beaker.
Explanation:
The distillation is the separation of two or more components from the mixture for volatile compounds. In this method, we employ the difference in the boiling point of different compounds. A compound with low boiling point evaporates fast. We collect these volatile compounds using condenser.
With the thermometer, we control the temperature so that we receive only one compound in the condenser.
This is a costly method as it requires enormous water to cool the condenser and costly setup. We use it only in the separation of precious products where we need all the components; like the perfume industry, petroleum refinery, some distilled beverage, in treating water salinity.
In general, purpose where the component is not costly, we use simple boiling and let the water escape.
Facts about acetone:
Acetone is a colourless organic compound. We use it in the lab to clean lab equipment as it dissolves many chemicals. We can see its use as a nail polish remover in daily life.
Next: Separation of impurity by crystallisation, Activity 2.9.
See also: Separation of a mixture using chromatography, Activity 2.7.
dis vido is ver y nicw
This is very helpful for me.Thanks a lot.