Table of Contents
Activity 1.1 Explanation NCERT Science Class 9 Matter In Our Surroundings
Brief procedure:
Activity 1.1 asks us to dissolve some amount of salt in water and observe the change in the volume.
Observation:
There is no change in the volume of water.
Explanation:
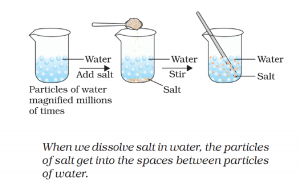
Further explanation by example:
You can compare water like a sponge. There are a lot of empty spaces in them. Any smaller substance takes those space without changing the shape or volume of sponge.
Related Facts:
- Solubility in water depends on this feature. If a particle can sit in those spaces, the object is soluble. If the particle cannot accommodate in those voids, they do not dissolve and settle to the bottom.
- Change in volume is not constant after adding too much salt. After a certain point, the salt molecule fills all the empty voids. Now there is no space for any other salt molecule. Beyond this stage, salts deposit on the bottom and volume of water increases. We call this stage a Saturation point. The saturation point of a substance is fixed for a particular liquid. For example, the saturation point of salt at room temperature in the water is 357 gm/litre.
Next: Addition of potassium permanganate in water: activity 1.2.
See also:
Activity list Chapter 1 Matter In Our Surroundings.
Can u plz explain the activities of 1.2 1.6(a), 1.7 ,1.8, 1.9 .
Thank you for sharing this . this is very useful to me
hello, how can i solve this problem with this page showing? eyeg
CAN YOU PLEASE EXPLAIN THE CONCLUSION FOR ACTIVITY 1.1
It’s really helpful. Thanks for sharing this information