A metal compound forms effervescence Dil. HCl. This gas extinguishes the fire. What is its formula if one of the products of the reaction in calcium chloride?
Answer:
Ca(X) +2HCl —–> CaCl2 + CO2
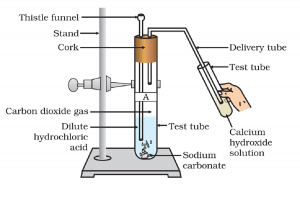
All metal carbonates and hydrogen carbonates(bicarbonate) react with acids and produce carbon dioxide gas (Activity 2.5). In such reactions metal carbonate from salt, with acids along with carbon dioxide. Here salt is calcium chloride. So, the compound is calcium carbonate. Calcium carbonate reacts with dilute hydrochloric acid and forms carbon dioxide. Carbon dioxide does not react with oxygen. So, it is used as fire retardant or fire extinguisher.
CaCO3(s) + 2HCl(aq) —> CaCl2 (aq)+ CO2(g)
Next: Acids like HCL, HNO3, H2SO4 .. etc are acidic but not the alcohols and glucose. Why?
See also: Why a reaction of a metal with acid liberates gases.
Very useful explanation.