Table of Contents
Activity 2.9 NCERT Class 10 Science, Chapter 2 Acids, Bases, and Salts
Procedure:
Activity 2.9 asks us to react solid sodium chloride with concentrated sulphuric acid and check if the gas evolved turn the blue litmus paper into red or not.
Observation:
Gas fumes produce but do not turn the blue litmus paper into the red.
Explanation:
Solid sodium chloride reacts with concentrated sulphuric acid and forms dry hydrochloric acid gas. Here HCl is produced in gaseous form as there is no water.
NaCl(S) + H2SO4(Conc.) ———> Na2SO4 (s)+ HCl(g)
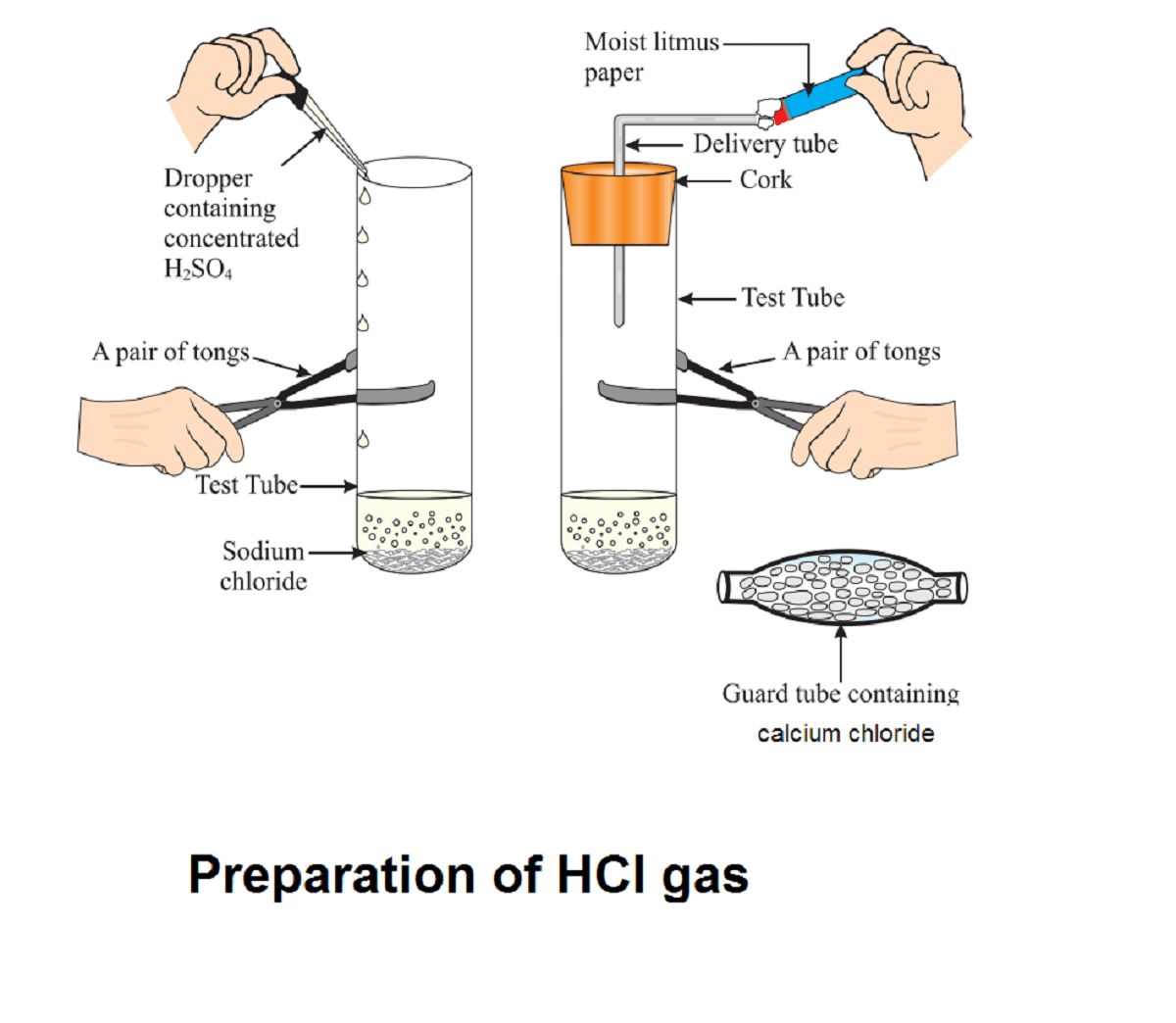
A litmus paper works on H+ or OH- ion. Since there is no water in HCl gas, HCl does not dissociate into its ion and does not turn the blue litmus red.
HCl (aq) ——-> H+ + Cl–
Inference/Conclusion:
Concentrated acids and bases need an aqueous medium to turn litmus paper.
Fact:
If we use moist blue litmus paper (as depicted in the diagram) then HCl gas will turn blue litmus paper into the red.
Next: Activity 2.10: Dissolution of concentrated sulphuric acid in water produces heat,
See also: Activity 2.8: Acids, bases, and salt increase the electrical conductivity of the water.
Other activities from NCERT class 10 science Chapter 2.
Reference: NCERT textbook for class 10 Science.
Help me a lot
Nice
good explanation
555
Thank youu so muchh!
It helped a lot
Nice
It helped a lot
Thanks, it helped a lot
Nice job guys
Well explained.
Nice Explanation !
Very helpful
Nice explanation
very nice and helpful thx…..