Activity 2.8 NCERT Class 10 Science, Chapter 2 Acids, Bases, and Salts
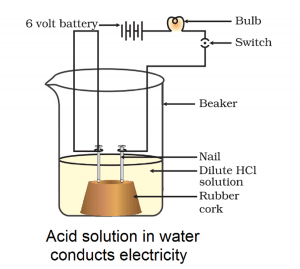

The activity 2.8 asks us to pass electricity through the various solution and see if bulb glow.
Observation:
The bulb glows with Hydrochloric acid and sulphuric acid but does not glow with alcohol or glucose solution.
Explanation:
Acid ionises in water solution. As a result, ions are freely available in the water to conduct electricity. As a result, bulb glow. Alcohol and glucose are organic compounds. They do not ionise in water. As a result, such a solution does not conduct electricity.
Inference/conclusion:
Acids, bases and salts increase the electrical conductivity of the water.
Next: Reaction of Metal salts with concentrated sulphuric acid, Activity 2.9.
See also: Metal oxides react with acids to form metal salts, Activity 2.7.
Note:
All salts, strong acids and strong bases ionise the water and hence increases the conductivity.
THANKS
It is written in NCERT that”Repeat this activity using alkalies such as sodium hydroxide, calcium hydroxide,etc. What can you conclude from the results of this activity” But you forget mention it after Activity 2.8 in Class X.
Thank you