Activity 2.4 NCERT Class 9 Science Chapter 1 Is Matter Around Us Pure
Brief procedure:
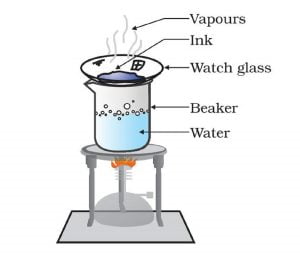
Activity 2.4 asks us to heat the solution of ink and water.
Observation:
On heating the solution, water evaporates, and we get back the ink dye in the watch glass.
Explanation:
The different substance has a different boiling point. We use this property to separate the components of the mixture. Here, the boiling point of ink is much higher than that of water. On heating the ink solution, water evaporates while ink dye remains in the china dish.
Application:
Crude petroleum refinery: Crude Petrol obtained from the earth contains a mixture of various petroleum products like petrol, diesel, LNG, vaseline etc. All these components have a different boiling point. This difference helps us in separating them.
Separation of metals from its alloys in metallurgical industries.
Preparation of milk powder/khoya from milk.
Next: Separation of a mixture by centrifugation, Activity 2.5.
See also: Experiment to demonstrate solubility increases with temperature, Activity 2.3.
There will only the Dye of ink remain and water gets evaporated from it.
To obtain dye from ink
whay is the aim
Įņķ
No,ink
There is the ink dye left in the watch glass
Is there a residue on watch glass?
Because ink is a mixture of water to separate ink and water we have to heat ink
Why we don’t heat ink directly