Activity 2.15 Class 10 Science, Chapter 2 Acids, Bases, and Salts
Brief Procedure:
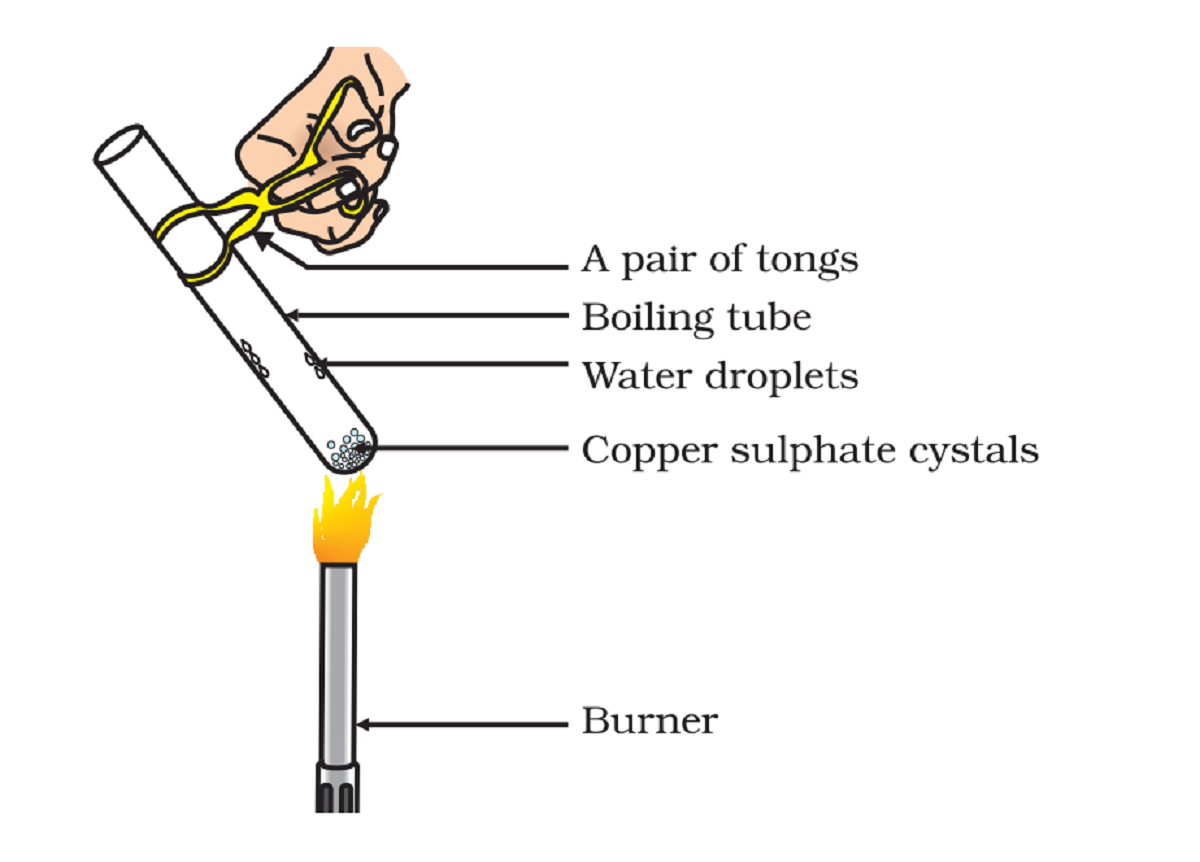
Activity 2.15 asks us to heat copper sulphate and then add water to it and observe the change in the colour.
Observation:
Copper sulphate is usually blue when kept in a room.
- On heating, copper sulphate becomes colourless and we also see a few drops in the test tube.
- When we add water to the heated test tube, copper sulphate restore its colour and become blue again.
Explanation:
Copper sulphate absorbs moisture from the air and forms its hydrate (CuSO4.5H2O). The hydrate of copper sulphate is blue. So, we also call copper sulphate as blue vitriol.
When we heat copper sulphate its water of hydration goes away, and the substance becomes colourless.
When we add water, dehydrated copper sulphate regains its water of hydration and becomes blue.
Inference:
This experiment shows that
- a crystal which looks solid in normal condition may not be dry as well. It may contain some other molecules attached to it.
- These molecules may give the crystal different physical and chemical properties like the colour.
Next:
Activity explanation chapter 3 Metals and non-metals.
See also:
The solubility of salts and PH Activity 2.14
Other activities from Acid, Bases and Salts.
***Activity 2.15 Class 10 Science, Chapter 2 Acids, Bases, and Salts***
Really helpful and very easy to grasp the concepts
What is the inference of activity 2.14
Very good
Very good website to learn activitys or to study
great great king
Very good explaination, helped me a lot. A great n ausome
Great website. I really like this website.