Activity 2.1 NCERT Class 9 Science Is Matter Around Us Pure
Brief procedure:
Activity 2.1 asks us to:
- prepare various solutions with different amount of copper sulphate and compare them.
- Mix the different amount of copper sulphate and potassium permanganate solution and compare them.
Observation/findings:
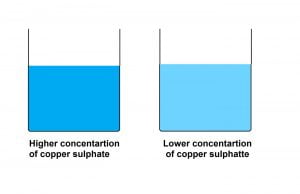
Different amount of copper sulphate in the solution gives different colour intensity.

Solute particles mix with the solvent and form a mixture. If there is only one type of solute particle, we call this mixture homogenous. If we add more than one type of particle, we call it a heterogeneous mixture. For example, any amount of copper sulphate in water is a homogenous mixture; while a mixture of copper sulphate and potassium permanganate in water is a heterogeneous mixture.
Colour intensity of a solution depends on the concentration of the solute and the solvent. If we add more solute particles in the solvent the colour intensity will increase. Another way to increase the intensity is by reducing the amount of the solvent eg, boiling the solution. In both of the cases the concentration of solute particle increases.
The mixture of potassium permanganate (red) and copper sulphate (blue) forms a mixture which has a different composition. Such a mixture is a Heterogenous solution.
Inference /Conclusion:
This experiment shows that a homogenous mixture can have variable colour intensity.
Some facts:
Air is a heterogeneous mixture of various gases like oxygen, nitrogen, carbon dioxide.
Thank you so much for the notes of this activity
Nice and Good explaination
Welcome
Thank you