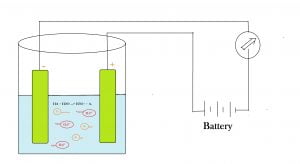
Why does an aqueous solution of acid conduct electricity?
Electricity is the movement of charge from one place to another place. movement of charges requires a medium. Acids in aqueous solution mean acid in water also called diluted acids. The polar nature of water splits the acid molecules into its cations and anions. Cations and anions are charged particles and are free to move inside the aqueous medium. As a result when a potential difference has applied the solution to conduct electricity.
HA + H2O —> H3O+ + A–
Facts:
Distilled water ( water without minerals, ions, etc ) does not conduct electricity.
Not only acids, but all salts and bases also make the aqueous medium more conductive. A pinch of salt and the solution become conductive.
Distilled water has a conductivity of 10-5 W-1m-1. While that of 0.1M HCL solution (3.65 gm in 1ltr water) has a conductivity of 0.41W-1m-1. This conductivity is 41000 times more than pure water!
A battery contains distilled water; normal water with traces of minerals and salts can make the battery useless.
Next: Why does dry HCl gas not change the color of the dry litmus paper?
See also: Acids like HCL, HNO3 are acidic but not the alcohols and glucose. Why?
***Why does an aqueous solution of acid conduct electricity***
Ref: NCERT Class 10 Science Chapter 3.