Question 3 Chapter 2 Is Matter Around Us Pure Class 9 Science
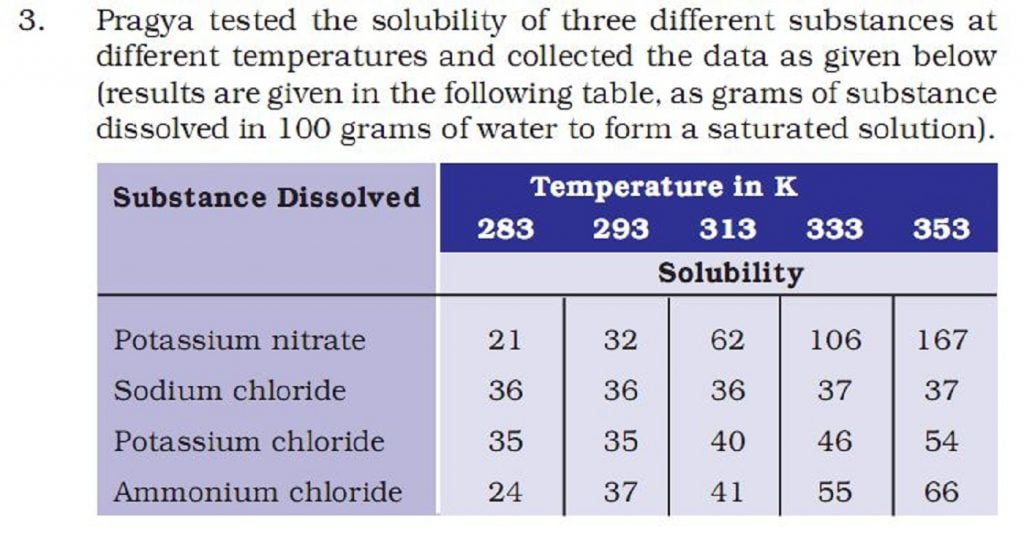
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
a) As per the table, it requires 62 gm of potassium nitrate per 100gm of water. So, for 50 gm of water, the required amount will be half of 62 i.e. 31gm.
b) solubility of potassium chloride at room temperature is only 35 gm. As a result, Potassium chloride solution becomes oversaturated on cooling. Here, only 35 gm will remain in the solution, and rest (54 – 35= 19 gm) form crystals of potassium chloride.
c) At 293K, the solubility of ammonium chloride is maximum.
d) With the increase in temperature, we also see an increase in the solubility.
Learning from this question:
- We see here that different salts have different solubilities, and the solubility increases with the temperature.
- The solubility of common salt (NaCl) increases negligibly with the change in temperature.
- On cooling a saturated solution, an oversaturated solution forms which do not dissolve. In the case of salts, they crystallise and sink to the bottom.
See also: Steps for making tea using terms solute, solvent, residue, filtrate.