Materials Metals and Non-metals MCQ Class 8 Chapter 4
Identify the non-metal here
- a. Sodium
- b. Zinc
- c. Carbon
- d. Copper
Which one of the following is not a characteristics of a metal?
- a. Malleability.
- b. Ductility.
- c. Good conductor of electricity
- d. Brittle.
An iron nail can be beaten into a sheet. The property is:
- a. Malleability.
- b. Ductility.
- c. Good conductor of electricity
- d. Brittle.
Metal used in home wire is:
- a. Copper
- b. Aluminium
- c. Silver
- d. Gold
Which metal remain liquid at room temperature
- a. Copper
- b. Aluminium
- c. Silver
- d. Mercurry
Which metal catches fire easily
- a. Copper
- b. Silver
- c. Mercury
- d. Sodium
In rusting iron read with which element ?
- a. Carbon dioxide
- b. Oxygen
- c. Nitrogen
- d. Helium
Metal oxides are
- a. Acidic
- b. Basic
- c. Neutral
- d. None
Metal oxides will turn which colour of litmus papaer
- a. Blue to red
- b. Red to blue
- c. Does not change the colour
Does other metals also rust in air
- a. Yes
- b. No
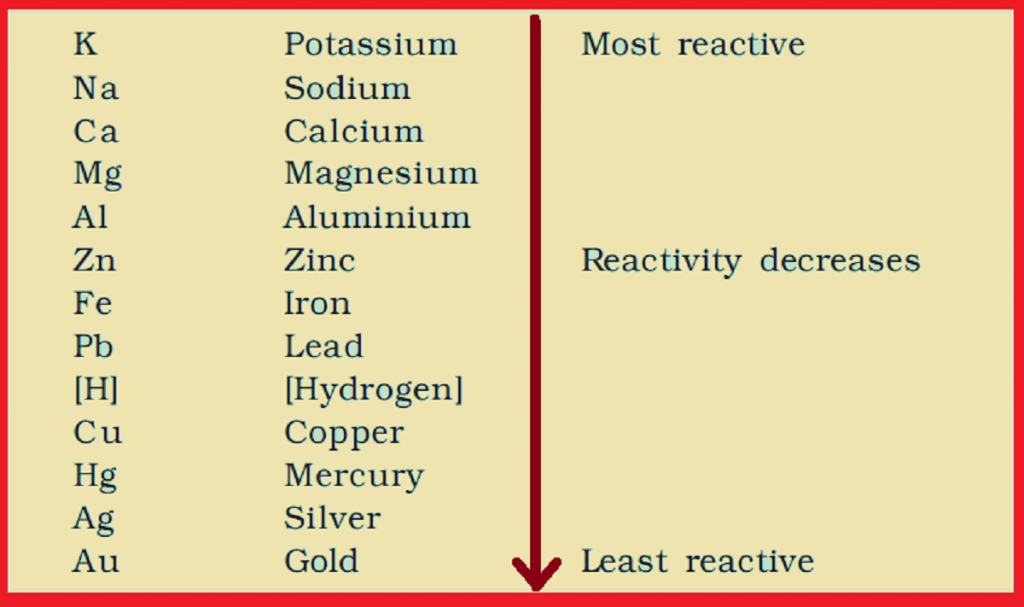
Highly reactive metals among these are
- a. sodium, potassium
- b. Silver, gold
- c. Copper, iron
- d. All three
Oxides of non-metal are
- a. Basic
- b. Acidic
- c. Neutral
- d. None
Metals reacts with acids to form salts. During this process which gas liberates?
- a. Hydrogen
- b. Chlorine
- c. Sulphur
- d. Oxygen
In double displacement reaction, which metal replaces another metal from its salt?
- a. Highly reactive metal
- b. Lesser reactive metal
- c. Inert metals
- d. None
See also:
Synthetic fibers and Plastics MCQ
Microorganisms Friend or foe MCQ
Crop Production and Management MCQ
Materials Metals and Non-metals MCQ Class 8 Chapter 4
Ref: Chapter 4.