Table of Contents
Activity 3.10 NCERT Class 10 Science, Chapter 3 Metals and Non-metals
Brief procedure:
Activity 3.10 asks us to react to various metals with water and observe the reaction.
Observation:
Reaction with cold water:
Metals like sodium and potassium vigorously react with water to form its oxide and hydrogen gas. Their reaction is so violent that hydrogen gas catches fire immediately.
2Na(s) + 2H2O(l) ———-> 2NaOH(aq) + H2(g)
2K(s) + 2H2O(l) ———-> 2KOH(aq) + H2(g)
Reaction with hot water:
Calcium is less reactive than sodium and potassium with cold water. They react spontaneously and emit hydrogen bubbles. But, the reaction is not so violent and hydrogen gas does not catch fire.
Ca(s) + 2H2O(l) ———-> Ca(OH)2(aq) + H2(g)
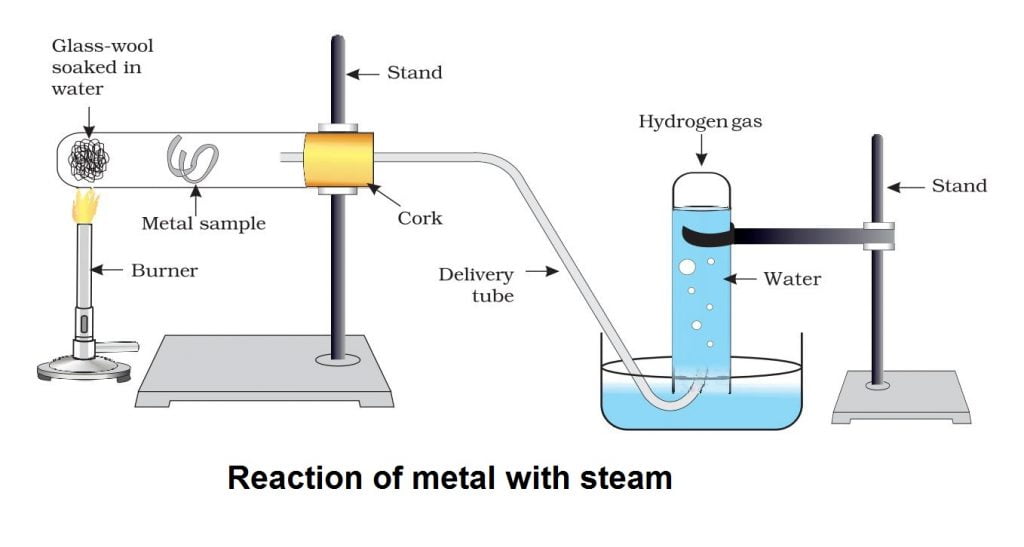

Metals like iron, zinc and aluminium do not react with cold or hot water. They react with steam to form corresponding hydroxide and hydrogen gas.
2Al(s) + 6H2O(l) ———-> 2Al(OH)3(aq) + 3H2(g)
No reaction:
Metals like lead, copper, silver and gold do not react with any form of water.
Order of reactivity with water in descending order:
Na>K>Ca>Zn>Fe>Al
Inference/conclusion:
Metals react with water and form metal hydroxide. It produces hydrogen gas, which we can check by placing a match-stick near it.
Next: See how various metals react with dilute hydrochloric acid — activity 3.11.
See also: Buring of metal in air, Activity 3.9.
Really helpful, well thoughtout content, thanks for this treasure! 10/10 recommended
Superb man ☠️
It’s his choice
Why lead doesn’t react with water or steam as it is more reactive than hydrogen
It was useful and was very helpful
Thank a lot
Was Very helpfull…. Plz cntnu doing this….
It was useful