Table of Contents
Activity 2.3 NCERT Class 10 Science, Chapter 2 Acids, Bases, and Salts.
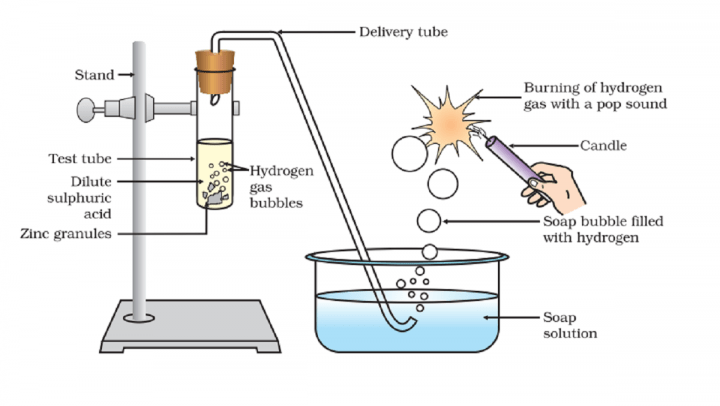
Brief procedure: Activity 2.3 asks us to react zinc granules with various acids and observe the flammability of gas formed.
Observation:
We see bubbles coming out vigorously with strong acids. This gas burn when we bring the candle to it.
Zinc also react with weak acids like acetic acid, but here gas formation is slow.
Explanation:
Zinc reacts with acids and forms its salt and hydrogen gas. Hydrogen gas liberates as the bubble. When we bring the flame to this gas, it burns as hydrogen is highly flammable.
For example
Zn + H2SO4 ———> ZnSO4 + H2
Weak acid does not dissociate quickly in water. So its reaction with zinc is slower as compared to strong acids like Hydrochloric acid and sulphuric acid.
Next: Reaction of zinc with bases. Activity 2.4.
See also:
Change in smell and colour of onion and vanilla with acid and bases. Activity 2.2.
Other activities from NCERT class 10 science Chapter 2.
Reference: NCERT textbook for class 10 Science.
On passing hydrogen gas through soap solution it does not get dissolved in it, and the solution easily form bubbles containing hydrogen gas.
formation of gas bubble takes place on surface of zinc granules
Bubbles are NOT formed in soap solution. It is formed when Zinc reacts with HCl. When Zinc reacts with the acid, it release Hydrogen gas as bubbles. It is then passed to soap solution.
Zn + H2SO4 —-> ZnSO4 (salt) + H2
Thanks sir
why are bubbles formed in soap solution?
It started reacts with sulphuric acid and bubbles of hydrogen gas is formed
Very poor explanation
What happens on the surface of the zinc granules ?
you haven’t given the answer of why bubble formed after passing the evolved gas through soap solution, but overall it was helpful.
Conclusion??
Not so useful
Ncy Answer
It is very helpful
Actually in the activity the gas that is formed is passed through soap solution in a dish with help of a tube. The gas which which comes through the soap solution will naturally create bubbles which is then shown to a burning candle. Then the bubble bursts with a pop sound which indicates the presence of hydrogen gas.
Ya, but it can’t make bubbles..
When hydrogen gas is allowed to pass through the soap solution then it will cause a agitation of formation of bubbles
because hydrogen gas which passes through soap solution helps to the formation of bubbles
Why are bubbles formed in the soap solution?
Why we can’t take water in place of soap solution.
Hydrogen gas is burn with pop sound in the activity
Bubbles are formed in the soap solution because hydrogen gas tries to escape from the solution as it doesn’t dissolve in the solution so when it reaches the surface bubbles are formed
Why are bubbles formed in soap solution?????