Table of Contents
Activity 1.9 Ncert Science class 10
Brief Procedure:
Activity 1.9 asks us to dip iron nails in a copper sulphate solution and check the colour of the solution.
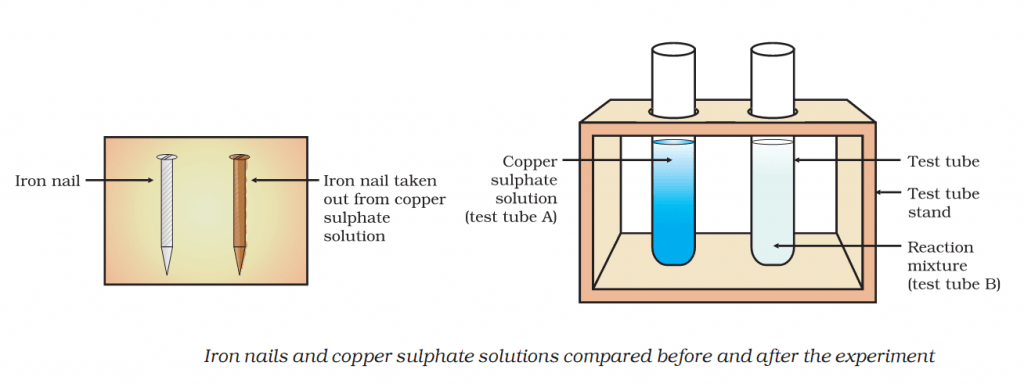

Colour of copper solution fades and nail becomes brown.
Explanation:
Iron in nail displaces copper from the copper sulphate solution. As a result, solution fades in colour.
This displacement reaction results in the formation of elemental copper. Elemental copper deposits on the nail and impart a brown colour to the nail.
CuSO4(aq) + Fe(s) ——-> FeSO4(aq) + Cu(s)
Note: The faded blue apperance later change to green colour because ferrous sulphate is green.
Next: Double Displacement reaction between Sodium sulphate and Barium chloride: Activity 1.10.
Read also:
Decomposition of silver chloride in sunlight. Activity 1.8.
Helpful thanks
He he he
Thank you this helped me !
Worthless Site
Please answer the questions that is given in NCERT activities.
Thanku
your studdy.in is very sensitive that complete my doubts