Activity 1.7 Ncert Science class 10
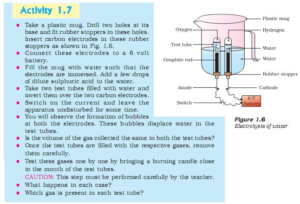
Procedure: The activity asks to electrolyse water using a 6-volt battery and check the volume of gases liberated at anode and cathode. The activity also asks to bring the gas at the flame and see what happens.
Observation:
- Gas collected at the cathode (-) is twice that of gas collected at the anode (+).
2. When we bring the flame to the gas collected at the cathode, it burns immediately. While gas obtained at anode does not burn.
Inference: Electricity decomposes water into its component. Hydrogen atoms in water receive an electron from the cathode electrode and form hydrogen gas. Similarly, oxygen atoms in water lose electrons at the anode and form oxygen gas.
Explanation:
We can explain this with the equation below.
2H2O ——> 2H2 + O2
Note: water is not highly conductive. We use a few drops of sulphuric acid here. It is done to increase the electrical conductivity of water. Instead of sulphuric acid, any other acid like hydrochloric acid or salt like sodium chloride can also work.
Next: Decomposition of silver chloride in sunlight. Activity 1.8.
Read also:
Decomposition of lead nitrate on heating. Activity 1.6.
Solved questions and activities of chapter 1 Chemical reactions and equations.
Activity 1.7 Ncert Science class 10
1 no. Bhai
Thanks ❤
Chapter 1 question & answers write in kannada medium
Helped a lot
Thanks a lot
Very helpful
As this activity is important, you guys made it very clear to understand thank you !!!!!
Your ans was fantastic thanks
Good great explanation
Not got the conclusion
you have explained me it just damn awsum wow very nice thank you for such a lovely answer
Nyc explained
why gas collected at cathode is twice gas collected at anode?
Great work !! Nice explanation in a simple and easy to understand language.