Atoms and Molecules class 9
4. Write the chemical formulae of the following.
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate.
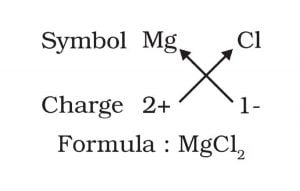
Answer:
The chemical formula of a compound balances the valance electrons. Magnesium has two valence electron while one chlorine atom needs one electron in its outer shell.
a) MgCl2, b) CaO, c) Cu(NO3)2, d) AlCl3, e) CaCO3
5. Give the names of the elements present in the following compounds.
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium Sulphate.
Answer:
a) Quick lime has a chemical formula of CaO. So, elements present in quick lime are calcium and oxygen.
b) Hydrogen bromide: HBr, Elements present here are hydrogen and bromine.
c) Baking powder is Sodium hydrogen carbonate (NaHCO3), so it contains sodium, hydrogen, carbon and oxygen element.
d) Potassium sulphate: (K2SO4). Here elements present are potassium, sulphur and oxygen.
Next: Question 6: calculation of molar mass.
See also: Question3. What are polyatomic ions? Give examples.